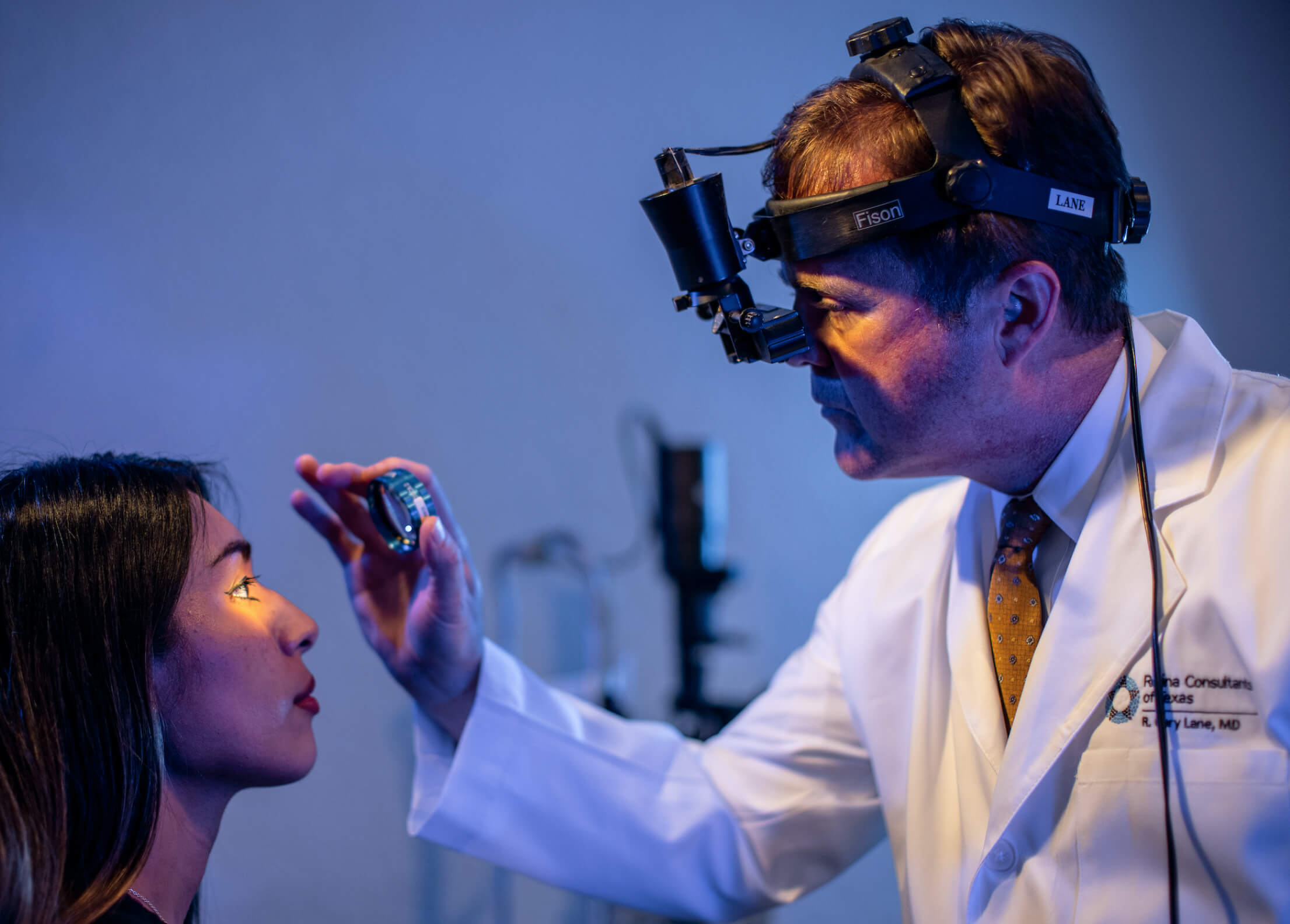
Partner with the global leader in retina research.
As the largest research network in the nation, we have the expertise, resources, and reach to lead Phase 1-4 studies. No matter the trial’s complexity, we maximize efficiency while maintaining quality.
A modern approach. Breakthrough results.
Your study will benefit from a powerful network of leading investigators, diverse participants, and advanced imaging equipment.
Therapeutic Expertise
- Diabetic Retinopathy
- Age-Related Macular Degeneration
- Geographic Atrophy
- Retinal Vein Occlusion
The RCA Value
- Average contracting completion < 35 days
- Study start-up < 80 days
- National Call Center to assist with recruiting potential subjects
- High-quality resources and best practices

Facilities built for Phase 1-4 clinical trials
Our 30,000 square-feet of space houses next-generation equipment necessary to execute your trial with precision, and we can work with you on additional technology integration if the study requires it. Featured Imaging Equipment:
- Heidelberg Spectralis OCT and OCT-A
- Optos California
- Standard 7 or 4 field FA and color fundus photos
- Microperimetry
- Octopus

A commitment to diversity and equity
Retinal health research is for the benefit of anyone in need of treatment, which is why it's crucial to recruit a wide range of participants across different ages, races, ethnicities, and genders. With an equitable approach, we can effectively get new medicines to more people.

We can get started quickly.
And we can scale.
A centralized, fully-integrated operational model allows us to activate studies quickly across site locations without compromising excellence.

Every standard of care starts by trying something new.
Connect with RCA Research today.




























